World Fertilizer, April 2020
Sulfuric acid (H2SO4) is the most common chemical
used for reducing pH during water treatment. A
colourless, odourless, and oily liquid, it is also
exceptionally corrosive due to its strong acidic
nature. In the 17th century, sulfuric acid was first produced
by Johann Glauber, who burnt sulfur together with
saltpetre (potassium nitrate, KNO3), in the presence of
steam. In various grades the acid is used in the
manufacture of fertilizers, pigments, dyes, drugs,
explosives, detergents, and inorganic salts and acids, as
well as in petroleum refining and metallurgical processes.
Manufacturing of sulfuric acid
In keeping with the general evolution of technology, gradual upgrading of the manufacturing of sulfuric acid has also taken place over time. There are two major processes used for the production of sulfuric acid: the lead chamber process and the contact process. However, the processes vary considerably from each other. A few basics that govern the manufacturing of the element are:
- Extraction of the pure element in the form of sulfur from its ore.
- Conversion of sulfur into sulfur dioxide (SO2) in the presence of oxygen (O2).
- Transformation of sulfur dioxide into sulfur trioxide (SO3).
- Conversion of sulfur trioxide into sulfuric acid.
Lead chamber process
The lead chamber process was first designed in 1746 by
John Roebuck of Birmingham, UK. The process has its
origin in the early preparation of sulfuric acid by the
oxidation of sulfur dioxide with nitric acid, but
produces sulfuric acid by oxidising sulfur dioxide with
moist air using gaseous nitrogen oxides as catalysts.
The reaction takes place primarily in a series of large,
box-like chambers of sheet lead that give the process
its name. It is an outdated process for the production
of sulfuric acid, but still continues to be practiced in a
few parts of the world. The summary of the process is
described by the equation below:
2SO2 + O2 → 2SO3
SO3 + H2O → H2SO4
During the process, hot sulfur dioxide is passed into
the lead chamber and then washed using nitrous vitriol.
Sulfur dioxide is then oxidised into sulfur trioxide.
Next, it is dissolved in acid wash leading to the
formation of glover acid, which is 78% sulfuric acid. In
the mean time, a mixture of gases, such as oxygen,
nitrogen, nitrogen oxides, sulfur dioxide and steam, are
passed from a glover tower through the lead chamber
where they react with water to produce sulfuric acid.
Contact process
The contact process is another method currently used
for the production of sulfuric acid in the high
concentrations required for industrial purposes.
Formerly, platinum was used as a catalyst for the
reaction, but as its reaction led to arsenic impurities in
the sulfur feedstock, vanadium oxide is now preferred
over platinum.
The contact process consists of five major steps:
Production of sulfur dioxide from sulfur
This can be achieved by burning sulfur in an excess of
oxygen to obtain a mixture of sulfur dioxide gas and
oxygen:
S + O2 → SO2
Or, by heating sulfide ores such as pyrites in an excess
of oxygen:
4FeS + 11O2 → 2Fe2O3 + 8SO2
Purification of sulfur dioxide
This removes dust particles, oxides of iron and arsenic. The
purification unit includes a series of stages:
- Dusting tower: the mixture of sulfur dioxide and excess of air contains several impurities that must be removed or risk a loss of efficiency from the catalyst. The sulfur dioxide is mixed with an excess of air and is passed through a purifier in which electric charges attract the solid particles that are removed.
- Washing tower: in this stage, sulfur dioxide is washed completely to rinse away any soluble contaminants.
- Drying tower: the gas is dried by a spray of concentrated sulfuric acid for further purification.
- CArsenic purifier: the gaseous mixture is directed into the arsenic purifier to remove arsenic oxide. Once the sulfuric dioxide is completely purified, it can be converted into sulfuric trioxide.
Converting sulfur dioxide into sulfur trioxide
This is carried out to manufacture industrial sulfuric acid, so
the contact process of sulfur trioxide production has to be
economically efficient. To convert sulfur dioxide to
sulfur trioxide, sulfur dioxide is mixed with oxygen and the
mixture is passed over a catalyst of vanadium oxide (V2O5) at
a temperature as high as 450˚C and at a pressure of 2 atm.
The mixture of sulfur dioxide and oxygen forms
sulfur trioxide. At standard conditions, the equilibrium is
positioned to the left and the amount of sulfur trioxide
produced is relatively small. In order to improve the yield of
sulfur trioxide, the reaction is carried out at 450˚C and 2 atm
using catalysts compounds such as V2O5.
2SO2 + O2 → 2SO3
Conversion of SO3 to oleum
In this step of the contact process, concentrated sulfuric acid
is used to dissolve the sulfur trioxide which produces the
oleum. Sulfur trioxide is cooled in a heat exchanger and is
then absorbed in concentrated sulfuric acid in another tower
to provide oleum with disulfuric acid (H2S2O7):
SO3 + H2SO4 → H2S2O7 – oleum
Dilution of oleum to sulfuric acid
In this final step of the contact process, dilution of oleum is
achieved by reacting it with water, and as a result,
concentrated sulfuric acid (98% H2SO4) is produced:
H2S2O7 + H2O → 2H2SO4
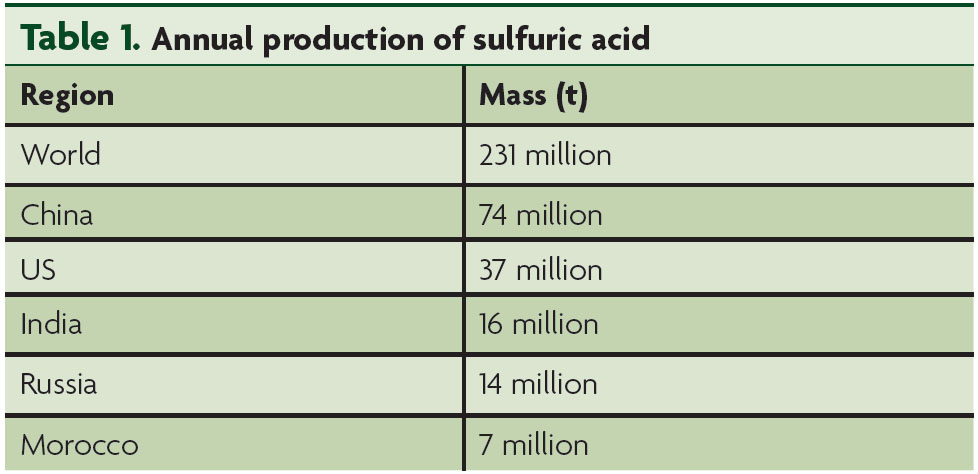
Annual global production of sulfuric acid
The global sulfuric acid market was valued at US$9.35 billion
in 2015 and is projected to reach US$11.10 billion by 2021, at a
compound annual growth rate (CAGR) of 2.87% between
2016 – 2021. The sulfuric acid market size was 244.26 million t
in 2015 and is expected to reach US$83 billion by 2022.
Fertilizer consumption is highest in China and India, followed
by the US. Based on calculations from the Food and
Agriculture Organization of the United Nations (FAO), the
caloric requirement is expected to double in the next
25 years, with the global population touching 8 billion. Sulfur
production is expected to increase by approximately 27%
between 2016 – 2021. In these respective studies, 2015 has
been speculated as the base year and 2021 as the forecast
year to estimate the market size of sulfuric acid. Prime drivers
include an increase in the production of nutrient-rich food
crops and steady and sustainable demand for sulfuric acid
due to its diversified applications.
Production of sulfuric acid in India
India, being one of the major producers of sulfuric acid in the
world, is characterised by a growing agriculture sector and
increasing metal processing activities, along with a rising
consciousness about wastewater treatment; each of these
factors drive the sulfuric acid market in India. According to a
study, the Indian sulfuric acid market is projected to exhibit a
CAGR of 3.35% between 2016 – 2025, on account of
increasing fertilizer production in the country. Moreover,
growing demand and consumption of sulfuric acid can be
attributed to the country’s rapid population growth, and is
slowly driving demand for infrastructure, food crops and
various base metals in the country. Sulfuric acid is prepared
for use as the most crucial raw material and main element in
the agricultural sector, with applications in production of
fertilizers and pH balance.
Case study: sulfuric acid plant in Saudi Arabia
Addar Chemicals Co., based in Jubail, Saudi Arabia,
contracted Nuberg EPC for the construction of a new
sulfuric acid and sulfolane acid plant in Jubail. The latter was
Saudi Arabia’s first greenfield sulfolane manufacturing plant
and has a capacity of 6000 tpd. The sulfuric acid plant has a
capacity of 80 tpd.
The scope of the project included process, technology
know-how, detailed engineering, design, procurement,
manufacture and supply of plant equipment as well as
erection, construction, project management, commissioning,
and start-up of the plant. The company followed local
regulations and standards as prescribed by the
Royal Commission for Jubail and Yanbu (RCJY) and the
Saudi Standards, Metrology and Quality Organisation (SASO).
Managing the process planning of both the plants at the
same time as well as at the same location was the main
challenge. Allocation of different sets of resources and
personnel between both plants would risk the project going
considerably over budget. To tackle this challenge, the
planning team put their full focus on the sulfuric acid plant
first and then, after the completion of that plant, moved on
to the allotment process of resources and staff in the
sulfolane acid plant’s planning stages. This kept costs low and
helped to deliver the project within budget.
Personnel management was again a challenge given
linguistic differences; however this issue was soon resolved,
with a total staff in peak time of 250 – 350. Due to visa
restrictions, the exiting of staff in a month at the time of
ongoing construction resulted in delays in executing
the project. Despite these challenges, the sulfuric acid plant
was completed on time.
Equipment/machinery
The scope of the project included the engineering,
procurement and construction (EPC) and lumpsum turnkey of
the sulfuric acid plant. An overview of some of the key
equipment and machinery for the project is presented below.
Detailed engineering package
Details about each individual component and process were
also stated with accompanying documents. This section
covered key engineering design features, such as:
- Process engineering: design basis, process flow diagram equipment list, process datasheet and line list.
- Piping engineering: design basis, piping layout, isometrics and bill of material.
- Rotary and static equipment: transformer, diesel generator set, uninterrupted power supply, battery charger and bus duct.
- Electrical and instrumental engineering: design basis, single line diagram, load list, cabling and earthing layout.
- Mechanical engineering: design basis, general arrangement equipment – static, design calculation, technical specification of rotator equipment.
- Fire and safety equipments: fire water pump, fire fighting systems (sprinkler system and extinguishers), fire alarm detection – heat detector, smoke detector, call point, very early smoke detection apparatus system.
- 3D simulation and software: Cadwork software was used to create a 3D model of the sulfuric acid plant, allowing it to be seen in in real time virtually, detect any issues and also provide a detailed knowledge of different disciplines on a single 3D model.
Project management
Some of the major key factors in the project management
processes for Addar included the following:
- Close monitoring of critical activities to ensure compliance.
- The use of bar charts and a critical path method (CPM) to indicate every activity of the project.
- Coordination with clients and vendors during the detailed engineering.
- Procurement and delivery progress control.
- Preparation of monthly progress reports with comments on compliance or deviations.
Conclusion
The sulfuric acid plant in Jubail was commissioned in November 2018 and executed by the company in Saudi Arabia. The plant produces sulfuric acid (98%), which is used for, among other purposes, the manufacture of ammonium sulfate and super phosphate of lime. In tandem with an increasing demand for organic chemicals the sulfuric acid industry has grown, with approximately 70% of today’s sulfuric acid production used for making fertilizers.
© This article was first published in World Fertilizer, April 2020.
Media Gallery
Chemical Engineering World - Healthy pipeline of projects
SiliconIndia - Nuberg EPC: Where Ideas Escalate to Expanses of Engineering Excellence
World Fertilizer - A World of Sulfur
International Cement Review - A new lime plant for Crescent
Process Worldwide - Making Ideas Happen, from Concept to Commissioning
World of Chemicals - EPC demand is rising in all dimensions of chemical industry
Financial Express - Indian firms can be world leaders in EPC
The Sunday Guardian - Engineering companies enthused by Make in India
Download PDF